Certifications & Accreditations
We have been certified by leading organizations to affirm the quality and reliability of our medical testing and research services.

Office of the Board of Investment (BOI)
Promoted Business Category:
Provision of medical testing services utilizing biotechnology (Category 7.12.6)
- Preparation of test results and/or synthesis of biological substances
- Quality assurance and control
- Verification of medical diagnostic accuracy
- Capability to expand testing services to handle up to 294,000 samples annually

Thailand Center of Excellence for Life Sciences (TCELS)
Confirmation of the enterprise’s eligibility under Category 7.12.6 – Biotechnology
Details of business activities in medical biotechnology:
- Genetic analysis through DNA sequencing
- Research and development of GeneChip technology (DNA Microarray)
- Personalized medicine diagnostics and analysis

Certificate of Registration – ISO 9001:2015
มาตรฐานที่ได้รับรอง
ISO 9001:2015 – ระบบบริหารคุณภาพ (Quality Management System)
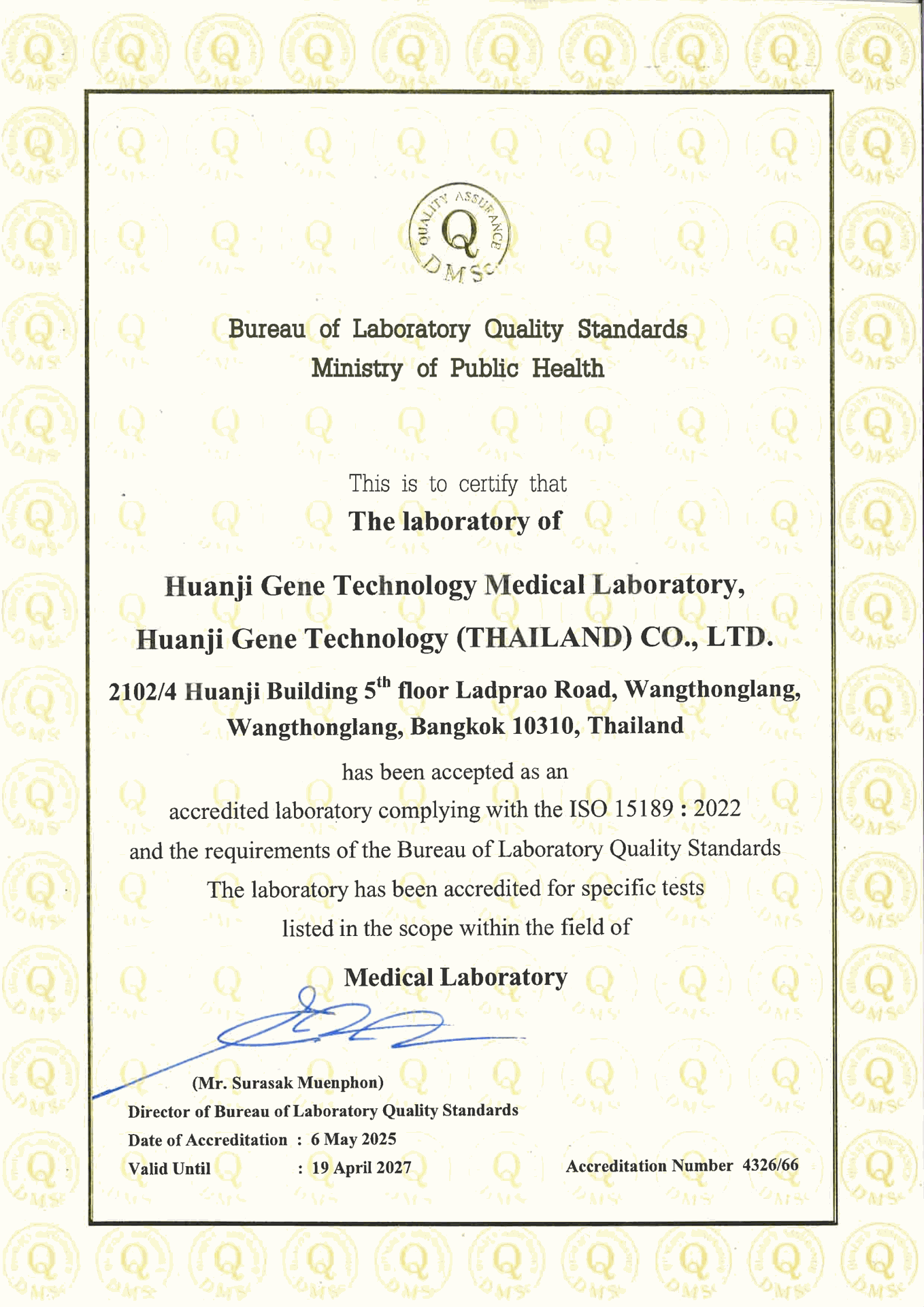
Accredited Laboratory Certificate – ISO 15189:2022
มาตรฐานที่ได้รับรอง
ISO 15189:2022 – ความสามารถเฉพาะด้านของห้องปฏิบัติการทางการแพทย์
(Medical laboratories – Requirements for quality and competence)

Certificate of Accreditation – ISO 15190:2020
มาตรฐานที่ได้รับรอง
ISO 15190:2020 – มาตรฐานด้านความปลอดภัยในห้องปฏิบัติการทางการแพทย์
(Medical laboratories – Requirements for safety)

Certificate of Laboratory Competency
for SARS-CoV-2 Detection by Real-time RT-PCR Method
Testing methodology includes:
Real-time RT-PCR
- Individual nasopharyngeal and throat swab sample
- Pooled nasopharyngeal and throat swab samples

Certificate of Laboratory Competency
for HPV Screening by HPV DNA Testing
Testing Method:
HPV DNA testing
Detection using reagents for 14 high-risk types of Human Papillomavirus (HPV)
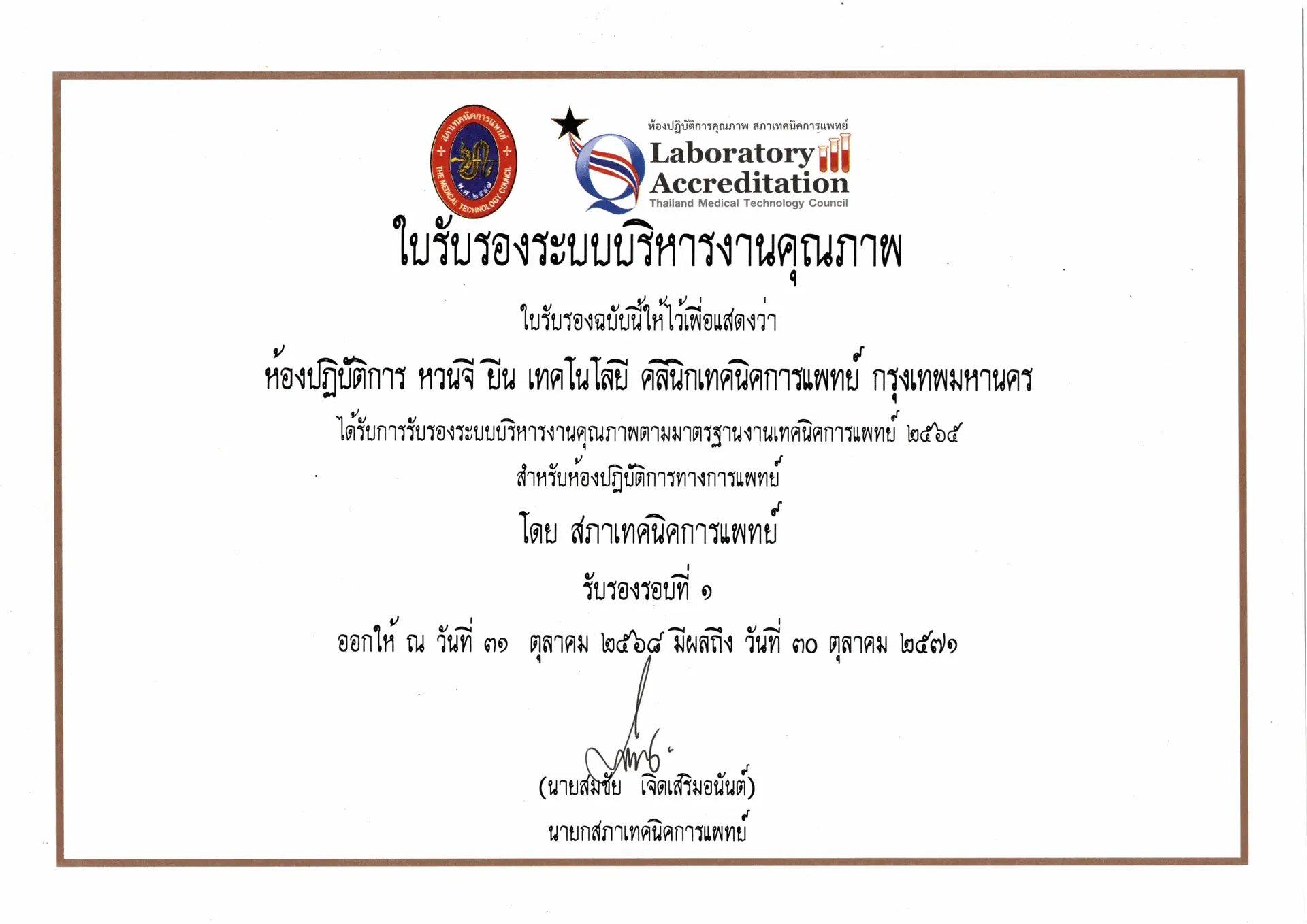
ใบรับรองระบบบริหารงานคุณภาพห้องปฏิบัติการ
โดย สภาเทคนิคการแพทย์ (ครั้งที่ 1)
มาตรฐานที่ได้รับการรับรอง
ตาม มาตรฐานงานเทคนิคการแพทย์ พ.ศ. 2565

Certificate of Technical Evaluation
Issued to:
Kunming Biochip Biotechnology Co., Ltd.
A certified medical laboratory
Laboratory Type:
Clinical PCR Laboratory for genetic material amplification

Yunnan Provincial Science and Technology Award Certificate
Issued to:
Kunming Biochip Biotechnology Co., Ltd.
Award Type:
Scientific and Technological Progress Award

Certificate of Medical Device Registration
Issued to:
Kunming Biochip Biotechnology Co., Ltd.
A registered manufacturer
Certified Product:
HPV diagnostic test kit utilizing Polymerase Chain Reaction (PCR) method

Computer Software Copyright Certificates
Issued to:
Kunming Biochip Biotechnology Co., Ltd.
Certified Product:
- Gene Analysis Software System
- DNA Test Result Management System
- Automated Pathogen Detection System
- Genetic Data Analysis System
- Software Platforms for Medical and Biotechnology Applications

High and New Technology Enterprise Certificate
Issued to:
Kunming Biochip Biotechnology Co., Ltd.
Details:
Certified as a “High and New Technology Enterprise” under the criteria of the Chinese government, reflecting the company’s strong capabilities in innovation, research and development, and the application of advanced biotechnology in its operations.

Specialized and Innovative Small and Medium-sized Enterprise (SME) Certificate
Issued to:
Kunming Biochip Biotechnology Co., Ltd.
Details:
Recognized as a “Specialized, Refined, Differential, Innovative” (SRDI) SME, demonstrating the company’s excellence in innovation, technological capability, and specialized expertise in the biotech industry.

Medical Device Manufacturing License
HPV Testing using HPV DNA Testing Method
Issued to:
Kunming Biochip Biotechnology Co., Ltd.
Scope of Manufacturing:
Class III Medical Devices – Registration Code: 6840
In Vitro Diagnostic Test Kits (In Vitro Diagnostic Reagents - IVD)
which includes:
- PCR Test Kits
- HPV Test Kits
- Infectious Disease Test Kits such as COVID-19, HIV, HPV, etc.

External Quality Assessment (EQA) Certificate
Issued to:
Kunming Biochip Biotechnology Co., Ltd.
Details:
The laboratory has successfully passed the national External Quality Assessment (EQA) for the year 2024 in the category of Nucleic Acid Detection for Non-viral Pathogens, with results rated as "Qualified".
- Mycobacterium tuberculosis DNA (TB DNA)
- Chlamydia trachomatis DNA (CT DNA)
- Neisseria gonorrhoeae DNA (NG DNA)

External Quality Assessment (EQA) Certificate
Issued to:
Kunming Biochip Biotechnology Co., Ltd.
Details:
Successfully passed the national-level laboratory quality assessment
- for HPV (Human Papillomavirus) genetic analysis,
- meeting official performance standards.

External Quality Assessment (EQA) Certificate
for Viral Nucleic Acid Testing
Issued to:
Kunming Biochip Biotechnology Co., Ltd.
Details:
Successfully passed the national-level laboratory quality assessment
- Quantitative HBV DNA testing
- Quantitative HCV RNA testing

